Recessive Genetic Conditions of Wagyu Cattle
Table of Contents
Recessive genetic conditions in Wagyu cattle are inherited and having one that is a carrier of such condition is fairly common. Being a carrier doesn’t mean that they are affected by the condition but we do recommend that you put them in a proper breeding plan so that the trait doesn’t get passed on to future generations.
Wagyu cattle are an excellent option for farming beef since they yield high-quality meat and have other desirable characteristics. Like any other breed of cattle, wagyu cattle are susceptible to hereditary disorders passed from parents to offspring. These disorders may impair their overall productivity, reproductive potential, and general health. Breeders and other interested parties must understand and deal with genetic disorders in Wagyu cattle to keep the breed alive and profitable. They can lower the risks of these disorders and keep the Wagyu breed’s integrity by doing thorough genetic tests and using smart breeding techniques.
Understanding Genetic Disorders
Mutations or variations in the DNA of Wagyu cattle cause genetic disorders that cause anomalies in development or physiological activities. These conditions can negatively impact the health and performance of the affected animals and can take many forms, from musculoskeletal abnormalities to metabolic diseases. Genetic abnormalities are traits passed down from parents to their offspring through genes from one or both parents. The disorders are caused by mutations in certain genes in charge of vital biological functions, including immune system function, muscle growth, or blood clotting.
Importance of Genetic Testing
In Wagyu cattle herds, genetic testing is essential for detecting carriers of genetic diseases. Breeders can identify animals with harmful genetic variations and stop the problems from being passed down to future generations using individual DNA analysis. Breeders can carry out selective breeding plans to lower the frequency of genetic diseases in Wagyu cattle while maintaining favorable features thanks to genetic testing. Breeders can efficiently regulate and reduce the impact of genetic disorders on the Wagyu breed by using alternative breeding tactics, such as gene editing or assisted reproductive technologies, or by carefully matching carriers with unaffected animals.
Reported Genetic Disorders in Wagyu Cattle
In Wagyu cattle, several prevalent genetic disorders have been identified, each with distinct characteristics and implications for animal health and productivity. Below are some of the known genetic recessive conditions in Wagyu cattle:
1. (B3) Band 3 Deficiency
B3 deficiency is characterized by hemolytic anemia, spherocytosis, splenomegaly, retarded growth, and acidosis. The transmembrane glycoprotein in red blood cells, The transmembrane glycoprotein in red blood cells is affected by this problem, which makes it harder for the body to facilitates ion transport, gas exchange, cell structure maintenance, cell adhesion, and enzyme regulation, essential for maintaining cellular homeostasis and proper physiological functioning (Inaba et al., 1996).
2. (F13) Bovine Blood Coagulation Factor XIII Deficiency
F13 deficiency causes very long bleeding times, which cause hemorrhages and hematomas and often result in death within days of birth..
3. (CHS) Chediak-Higashi Syndrome
CHS is an autosomal recessive disease marked by problems with platelets and a weak immune system. Cattle that are sick may have light coats and red eyes (Abdeen et al., 2013).
4. (CL16) Claudin-16 Defect
A condition characterized by impaired renal filtration and resorption. Hence, it is highly probable that the CL-16 protein plays a crucial part in the processes of filtering and resorption (Hiran et al. 2000).
5. (F11) Factor XI Deficiency
In Wagyu cattle is a hereditary disorder resulting in reduced blood clotting ability, leading to prolonged bleeding times and increased susceptibility to bleeding disorders. Carriers of the mutated gene may not display symptoms themselves but can pass the mutation on to their offspring (Matsumoto et al. 2023).
6. (IARS) Isoleucyl-tRNA Synthetase
This is the causative mutation for neonatal weakness with intrauterine growth retardation known as perinatal weak calf syndrome (Hirano et al., 2013). Calves with this syndrome exhibit such as anemia, depression, weakness, variable body temperature, astasia, difficulty nursing, growth retardation, and increased susceptibility to infection (Hiran et al. 2013).
Foundation animal carriers
Foundation animal carriers are the initial individuals or “foundation animals” that were discovered as carriers of various genetic diseases within the Wagyu cattle community. These animals are important because they possess genetic variations linked to specific disorders, which they may transmit to their offspring. Foundation animal carriers are essential for genetic testing and breeding programs that attempt to control and decrease the occurrence of genetic diseases in herds. Here is a list of Carriers that have been identified from the initial testing of foundation animals:
Chediak-Higashi Syndrome (CHS)
- Itomoritaka 002
- Itoshigefuji TF147
- Mazda
- Mitsuhikokura TF149
Claudin 16 Deficiency / Renal Tubular Dysplasia (CL16)
- Yasufuku Jr
- JVP Yasutanisakura 931
- Kimifuku TF726
Factor XI Deficiency (F11)
- Itohana 2 TF38
- Itoshigenami TF148
- JVP Fukutsuru 068
- Kitateruyasu 003
- JVP Yasutanisakura 931
- Terutani
- Kikutsuru Doi
- Kikushige TF150
- Kikuterushige
Isoleucyl-tRNA Synthetase Disorder (IARS)
- Fukutsuru J068
- Haruki II
- Itozurudoi TF151
- Kikutsurudoi TF146
- Kitateruyasudoi 003
- JVP Yasutanisakura 931
Spherocytosis (B3)
- JVP Yasutanisakura 931
Testing for Genetic Disorders
Here are some methods to test each disorder.
Band 3 Deficiency (B3)
- Genotyping: This method can focus on specific regions of the genome that harbor mutations linked to Band 3 deficiency.
- Marker-assisted Selection: Genetic markers associated with Band 3 deficiency can be used in breeding programs to avoid mating carriers and reduce the prevalence of the disorder.
Bovine Blood Coagulation Factor XIII Deficiency (F13)
- DNA Sequencing: Whole-genome sequencing can be employed to identify mutations in the Factor XIII gene linked to the deficiency.
- Genotyping: Specific genetic changes known to be connected to Factor XIII deficiency can be targeted using genotyping techniques.
- Marker-assisted Selection: Genetic markers associated with Factor XIII deficiency can inform breeding decisions to prevent the transmission of the mutation.
Chediak-Higashi Syndrome (CHS)
- Laboratory Assessment: Diagnosis involves laboratory tests to assess platelet function and immune system status.
- Clinical Evaluation: Symptoms such as light coats and red eyes are clinically evaluated.
- Genetic Testing: DNA sequencing or genotyping can identify mutations in the Lyst gene associated with CHS.
Claudin-16 Defect (CL16)
- Genetic Testing: DNA sequencing or genotyping can detect mutations in the CL16 gene linked to impaired renal filtration.
Factor XI Deficiency (F11)
- Coagulation Assays: Diagnosis involves coagulation assays to assess Factor XI activity.
- Genetic Testing: DNA sequencing or genotyping can identify mutations in the Factor XI gene associated with the deficiency.
Isoleucyl-tRNA Synthetase (IARS)
- Clinical Evaluation: Diagnosis involves clinical assessment for symptoms such as weakness and growth retardation.
- Laboratory Tests: Tests for intrauterine growth and susceptibility to infection may be conducted.
- Genetic Testing: DNA sequencing or genotyping can identify mutations in the IARS gene associated with perinatal weak calf syndrome.
The American Wagyu Association provides its members with various DNA testing conducted by a third-party DNA laboratory for the following disorders/ traits F11, F13, CHS, and B3, CL16 (Animal Registration, 2023)
Genetic Disorder Testing Status and Offspring Distribution Predictions
The genetic status of each tested animal will be reported as one of the four following results:
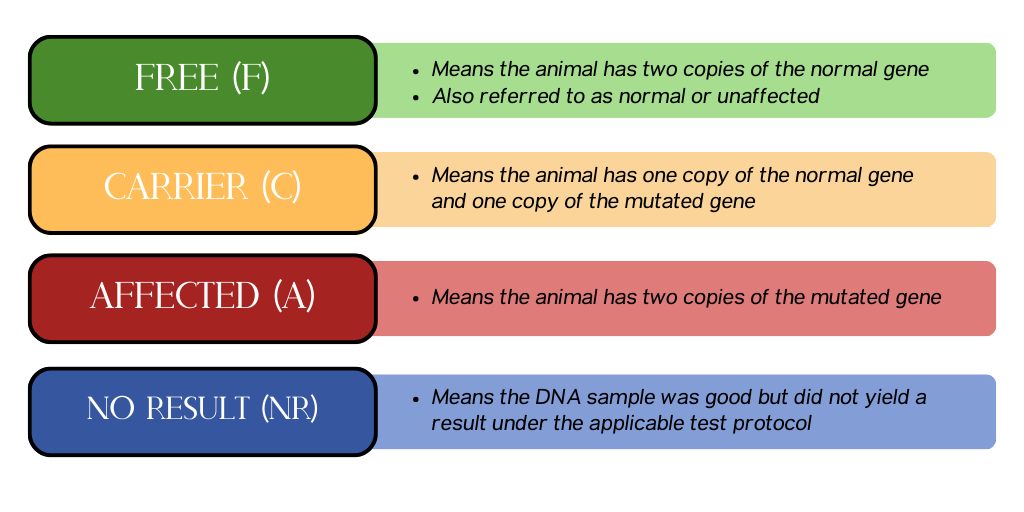
Free (F)
- Means the animal has two copies of the normal gene
- Also referred to as normal or unaffected
Carrier (C)
- Means the animal has one copy of the normal gene and one copy of the mutated gene
Affected (A)
- Means the animal has two copies of the mutated gene
No Result (NR)
- Means the DNA sample was good but did not yield a result under the applicable test protocol
The Science of Genetics Predicts the Following Results from Each Type of Mating:
Managing Recessive Genetic Disorders
For proper herd management, breeders need to have an accurate understanding of the status of their cattle with respect to the genetic disorders. Without knowing the Free, Carrier, and/or Affected cattle in a breeder’s herd, it is impossible to eliminate or reduce the risk of propagating the disorders in future generations of cattle.
Methods for managing recessive genetic disorders are breeder-specific and depend on the type of cattle operation, i.e., registered, commercial, fullblood, purebred, percentage, seedstock, beef production, etc. Below are some suggested methods for breeders to consider.
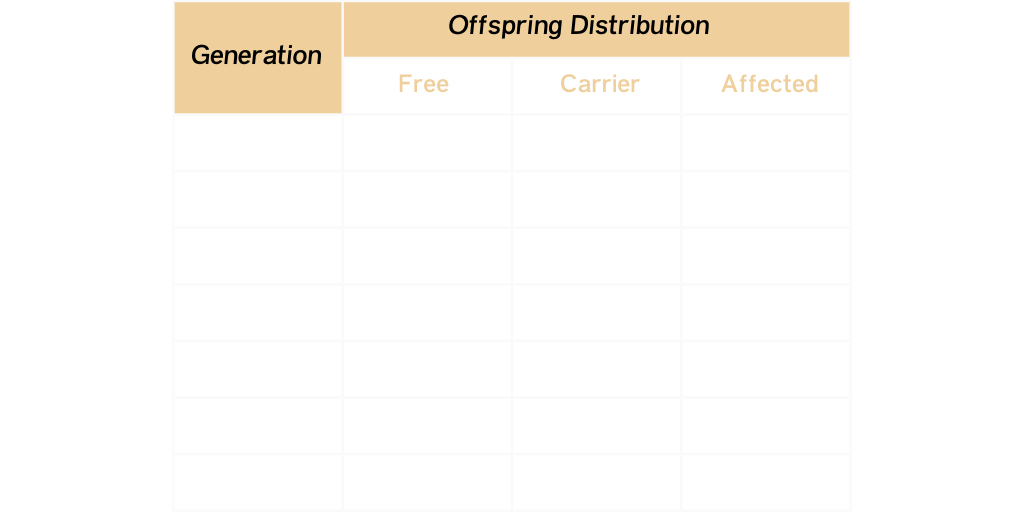
1. Test all animals and remove Affected animals from the herd. Always use Free animals to mate with any Carrier animals remaining in the herd. A commitment must be made to test all offspring from Carrier animals that will remain in the breeding herd. The Carrier rate will be reduced over time in future generations.
This table demonstrates the reduction in the Carrier rate in future generations when consistently using Free sires on Carrier dams and their future generational offspring.
As you can see the Carrier percentage is basically eliminated by the seventh generation and no Affected cattle are in the offspring distribution.
2. Test all animals and remove all Affected animals and Carrier sires. Use only Free sires in the breeding program going forward. A commitment must be made to test all offspring from Carrier dams that will remain in the breeding herd.
3. Test and remove Carrier and Affected animals from the herd. Only use Free animals in the breeding program going forward. No further testing will be required. This method will ensure a totally Free herd going forward.
4. Test all animals and use Carrier and/or Affected animals as recipients. If a cleanup bull is used, it should be Free. Offspring DNA verified to the cleanup bull must be tested for any animals that will remain in the breeding herd.
Why breeding practices are important
Emphasizing Responsible Breeding Practices: Responsible breeding practices are crucial for minimizing the occurrence of genetic disorders in Wagyu herds. This means doing a full pedigree study, keeping detailed records of how Breeding turned out, and putting animals’ health and safety first when making breeding choices. Breeders can ensure the genetic health of their Wagyu cattle herds and create successful breeding programs by following these guidelines.
Summary
It is ideal to address genetic disorders in Wagyu cattle to guarantee the herd’s health, welfare, and long-term viability. Breeders can efficiently control these disorders while maintaining desirable traits within the population. Wagyu populations, especially in the United States, should address inbreeding due to the small gene pool in the country. By collaborating with experts and implementing responsible breeding practices, breeders can safeguard the genetic health of Wagyu herds and promote the long-term success of their breeding programs. Ultimately, these efforts contribute to the continued prosperity of the Wagyu breed, supporting sustainable agriculture and providing consumers with high-quality products.
FAQs
Wagyu cattle can have health problems, be less productive, and sometimes could be fatal,. Because Wagyu communities outside of Japan don’t have a lot of different genetics, the chance of genetic diseases is higher. This makes proactive management methods very important.
For Wagyu cow herds to find carriers of genetic diseases, genetic testing is optional. By looking at each person’s DNA, breeders can make smart decisions about when to breed to keep the genetic purity of the group and lower the risk of passing on genetic diseases to future generations.
Genetic disorders can be managed in a number of ways by breeders. These include selective breeding to lower the number of deleterious alleles, removing carrier animals from the breeding population, and working with experts like geneticists and veterinarians to come up with good breeding plans.
Genetic testing options for Wagyu cattle are marker-assisted selection. Breeders can use this techniques to find specific genetic variants linked to known genetic diseases. This helps them make breeding choices that will reduce the spread of these disorders in the population.
Responsible breeding practices for Wagyu cattle include carefully studying the animals’ ancestors, keeping accurate records of breeding results, and putting animals’ health and well-being first when making breeding choices. Breeders can protect the genetic health of Wagyu cattle herds and make sure their breeding programs are successful and last for a long time by following these rules.
References
Abbasi A., Khalaj M., Tsuji T., Tanahara M., Uchida K., Sugimoto Y. and Kunieda T., 2009: A mutation of the WFDC1 gene is responsible for multiple ocular defects in cattle. Genomics, 94(1), pp.55-62.
Abdeen A., Sonoda H., Kobayashi I., Kitagawa G. and Ikeda M., 2013: A New Method for Rapid Detection of the Mutant Allele for Chediak-Higashi Syndrome in Japanese Black Cattle. Journal of Veterinary Medical Science, 75(9), pp.1237-1239.
American Wagyu Association. (2022, September 13). Testing for Inherited Recessive Genetic Disorders for U.S. Red and Black Wagyu Cattle: A Fact Sheet and Guide for Producers (Revised). [PDF document]. American Wagyu Association. Retrieved from https://s3.us-east-1.amazonaws.com/assets.wagyu.org/inherited-recessives.pdf
Hirano T, Kobayashi N, Matsuhashi T, Watanabe D, Watanabe T, Takasuga A, et al. (2013) Mapping and Exome Sequencing Identifies a Mutation in the IARS Gene as the Cause of Hereditary Perinatal Weak Calf Syndrome. PLoS ONE 8(5). [online] Available at: https://doi.org/10.1371/journal.pone.0064036
Hirano T, Kobayashi N, Itoh T, Takasuga A, Nakamaru T, Hirotsune S, Sugimoto Y. Null mutation of PCLN-1/Claudin-16 results in bovine chronic interstitial nephritis. Genome Res. 2000 May;10(5):659-63. doi: 10.1101/gr.10.5.659. PMID: 10810088; PMCID: PMC310863
Inaba M., Yawata A., Koshino I., Sato K., Takeuchi M., Takakuwa Y., Manno S., Yawata Y., Kanzaki A., Sakai J., Ban A., Ono K., and Maede Y., 1996: Defective anion transport and marked spherocytosis with membrane instability caused by hereditary total deficiency of red cell band 3 in cattle due to a nonsense mutation. Journal of Clinical Investigation, 97(8), pp.1804-1817. (in Japanese)
Watanabe D., 2017: Current topics in nationally designated defective hereditary traits of Japanese Black cattle – isoleucyl-tRNA synthetase deficiency, Bartter syndrome and forelimb-girdle muscular anomaly -. Japanese Journal of Large Animal Clinics, 8(1), pp.1-6. (in Japanese)
Tsujimoto, M. (2019). Literature Review on Establishment of Japanese Black Cattle (Wagyu) and Future Prospects of Its Industry [Master’s thesis, Unpublished]. Retrieved from http://hdl.handle.net/10832/2364
Scraggs E., Zanella R., Wojtowicz A., Taylor J.F., Gaskins C.T., Reeves J.J, de Avila J.M., & Neibergs H.L. (2014) Estimation of inbreeding and effective population size of full-blood wagyu cattle registered with the American Wagyu Cattle Association. Journal of Animal Breeding and Genetics 131, 3–10, doi 10.1111/jbg.12066.
Sasaki, S., Hasegawa, K., Higashi, T., Suzuki, Y., Sugano, S., Yasuda, Y., & Sugimoto, Y. (2016). A missense mutation in solute carrier family 12, member 1 (SLC12A1) causes hydrallantois in Japanese Black cattle. BMC Genomics, 17(1). doi:10.1186/s12864-016-3035-1
Matsumoto, H., Kimura, S., Saito, R., Takeichi, M., Kashimura, A., & Inenaga, T. (2023). Causative alleles for chondrodysplastic dwarfism, factor XI deficiency, and factor XIII deficiency in the Kumamoto sub-breed of Japanese Brown cattle. Animal Science Journal, 94(1). https://doi.org/10.1111/asj.13882
Bennett, S. (2013) History of Wagyu exports from Japan. Wagyu International. http://www.wagyuinternational.com/global_Japan.php. Accessed 2/14/22.
Animal Registration. (2023, August 28). American Wagyu Association. https://wagyu.org/for-members/registration